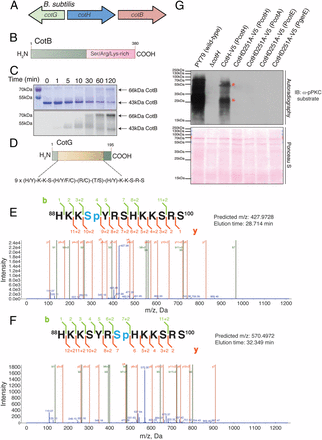
B. subtilis CotH phosphorylates the spore coat proteins CotB and CotG. (A) B. subtilis cotH gene forms a cluster with two genes that encode spore coat proteins cotB and cotG. (B) Schematic representation of B. subtilis CotB depicting the Ser/Arg/Lys-rich C-terminal region. (C) Time-dependent incorporation of 32P from [γ-32P]ATP into 6× His-tagged CotB by B. subtilis CotH. Reaction products were separated by SDS/PAGE and visualized by Coomassie staining (Upper), and radioactivity was detected by autoradiography (Lower). (D) Schematic representation of B. subtilis CotG depicting the nine tandem repeats in the protein. Representative tandem mass spectroscopy (MS/MS) fragmentation spectra depicting Ser4 (E) and Ser7 (F) phosphorylation of CotG(88–100) by CotH is shown. (G, Upper) Protein immunoblotting of B. subtilis spore coat extracts using a phosphospecific PKC substrate antibody depicting the phosphorylation of spore coat proteins in the different strains. (G, Lower) Ponceau S-stained membrane is shown as a loading control. The asterisks depict proteins that we infer are CotB and CotG based on their molecular masses.





