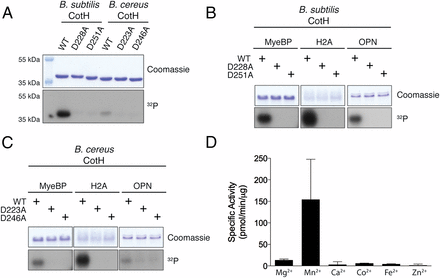
B. subtilis and B. cereus CotH are active protein kinases. (A) Incorporation of 32P from [γ-32P]ATP into B. subtilis and B. cereus wild-type (WT) CotH or the indicated mutants. Reaction products were separated by SDS/PAGE and stained with Coomassie blue. The incorporated radioactivity was visualized by autoradiography. Incorporation of 32P from [γ-32P]ATP into MyeBP, H2A, and OPN by B. subtilis wild-type CotH or the indicated mutants (B) and B. cereus wild-type CotH or the indicated mutants (C). Reaction products were analyzed as in A. (D) Incorporation of 32P from [γ-32P]ATP into a peptide substrate, CotG(88–100), which is derived from the CotH substrate CotG (described in Fig. 6) by B. subtilis CotH. The reactions were carried out in the presence of 5 mM MgCl2, MnCl2, CaCl2, CoCl2, FeCl2, or ZnCl2, and the reaction products were spotted on P81 phosphocellulose filter papers and terminated by immersion in H3PO4. Filter papers were washed, and incorporated radioactivity was quantified by scintillation counting.





