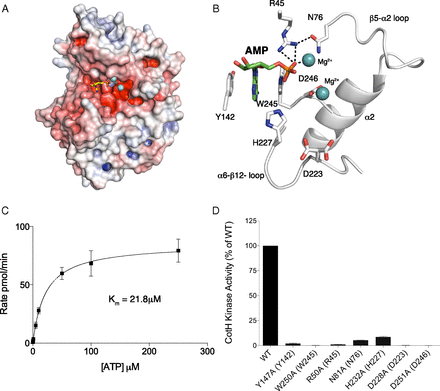
Mg2+/AMP-bound structure of B. cereus CotH reveals a unique mode of nucleotide binding and highlights residues involved in catalysis. (A) Surface representations illustrating the electrostatic potential of B. cereus CotH depicting AMP (yellow sticks) and Mg2+ (cyan spheres) bound in a cleft between the N- and C-lobes of the kinase. The electrostatic potentials were calculated with the APBS2.1 plug-in of PyMOL (65). The gradients of electrostatic potentials shown ranged from ≥−5 kbT/ec (red) to ≤+5 kbT/ec (blue), where kb is Boltzmann’s constant, T is temperature in degrees Kelvin, and ec is the charge of an electron. (B) Expanded image of the nucleotide-binding pocket showing the detailed molecular interactions important for nucleotide binding and catalysis. The AMP molecule and the two Mg2+ ions are shown as green sticks and cyan spheres, respectively. The salt bridge and hydrogen bond interactions are shown as dashed lines. (C) Kinetic analysis depicting the concentration dependence of Mn2+/ATP on the rate of phosphate incorporation into CotG(88–100) by B. subtilis CotH. (Inset) Km for Mn2+/ATP is indicated. Reaction products were analyzed as in Fig. 2D. (D) Kinase activity of B. subtilis CotH and active site mutants was analyzed as in Fig. 2D. Activity is expressed relative to activity of the wild-type enzyme. The amino acids in brackets indicate the corresponding residues in B. cereus CotH.





