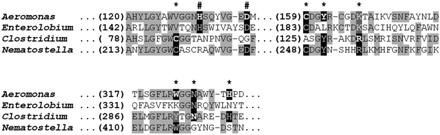
Conservation of functional residues in aerolysin eukaryotic homologues. Multiple sequence alignment of aerolysin domains of aerolysin from the bacterium Aeromonas hydrophyla (GenBank accession 1pre_A), enterolobin cytotoxin from the plant Enterolobium contortisiliquum (Swiss-Prot accession P81007), Clostridial α-toxin from the bacterium Clostridium septicum (GenBank accession Q8GI65) and an aerolysin homologue from the sea anemone Nematostella vectensis (NCBI reference XP_001634534). Conserved positions appear in black font on light gray background. Positions proved by mutagenesis to be involved in activity, are indicated by asterisk (MacKenzie et al. 1999; Kennedy et al. 2009). Two positions previously shown to be involved in oligomerization of aerolysin are indicated by the pound sign (Buckley et al. 1995). The position originally mutated appears in bold, conserved residues at these positions appear on black background, and conservative substitutions are in white font on dark gray background.




