Protas, Anna Maria and Ariani, Hanieh Hossein Nejad and Bonna, Arkadiusz and Polkowska-Nowakowska, Agnieszka and Poznański, Jarosław and Bal, Wojciech (2013) Sequence-specific Ni(II)-dependent peptide bond hydrolysis for protein engineering: active sequence optimization. Journal of inorganic biochemistry, 127 . pp. 99-106. ISSN 1873-3344
|
Microsoft Word
614kB | ||
|
Image (TIFF)
107kB | |
|
Image (TIFF)
70kB | |
|
Image (TIFF)
93kB | |
|
Image (TIFF)
838kB | |
|
Image (TIFF)
379kB | |
|
Image (TIFF)
403kB | |
![[img]](http://eprints.ibb.waw.pl/631/20.hassmallThumbnailVersion/Scheme1.jpg)
|
Image (JPEG)
64kB |
Official URL: http://www.sciencedirect.com/science/article/pii/S...
Abstract
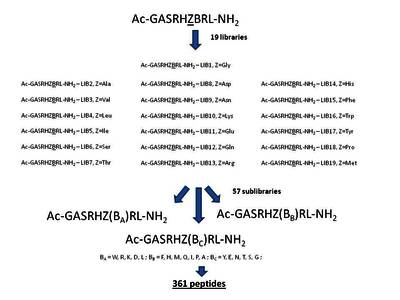
In previous studies we showed that Ni(II) ions can hydrolytically cleave a peptide bond preceding Ser/Thr in peptides of a general sequence RN-(Ser/Thr)-Xaa-His-Zaa-RC, where RN and RC are any peptide sequences. A peptide library screening, assisted by accurate measurements of reaction kinetics for selected peptides, demonstrated the preference for bulky and aromatic residues at variable positions Xaa and Zaa [A. Krężel, E. Kopera, A.M. Protas, A. Wysłouch-Cieszyńska, J. Poznański, W. Bal, J. Am. Chem. Soc., 132 (2010) 3355-3366]. In this work we used a similar strategy to find out whether the next residue downstream to Zaa may influence the reaction rate. Using an Ac-Gly-Ala-Ser-Arg-His-Zaa-Baa-Arg-Leu-NH2 library, with Zaa and Baa positions containing all common amino acids except of Cys, we found a very strong preference for aromatic residues in both variable positions. This finding significantly limits the range of useful Xaa, Zaa and Baa substitutions, thus facilitating the search for optimal sequences for protein engineering applications [E. Kopera, A. Belczyk-Ciesielska, W. Bal, PLoS One 7 (2012) e36350].
| Item Type: | Article |
|---|---|
| Subjects: | Q Science > Q Science (General) Q Science > QD Chemistry |
| Divisions: | Department of Biophysics |
| ID Code: | 631 |
| Deposited By: | Prof Jaroslaw Poznanski |
| Deposited On: | 23 Jun 2014 08:23 |
| Last Modified: | 27 Oct 2014 15:04 |
Repository Staff Only: item control page